Severely ill patients will benefit from cutting-edge treatment under Early Access to Medicines Scheme
A new scheme means severely ill patients with life-threatening and seriously debilitating conditions, will be offered the lifeline of trying cutting-edge new medicine, years before it would normally reach them.
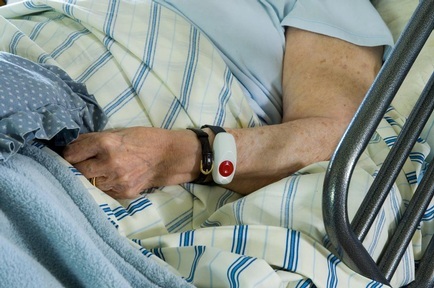
The Early Access to Medicines Scheme will enable these patients to benefit much quicker from innovative treatment. Under the scheme, doctors will work with patients to make innovative and promising drugs available as soon the Medicines and Healthcare Products Regulatory Agency - the UK’s regulator - has signalled that the benefits outweigh the risks following an initial scientific assessment.
Health Secretary Jeremy Hunt said: “Making Britain the best place in the world for science, research and development is a central part of our long term economic plan. This ground-breaking scheme will provide cutting edge medicines earlier, give hope to patients and their families and save lives. And as part of our strategy for Life Sciences it will create more jobs and opportunities for people, helping secure a better future for our country.”
Alongside the Early Access to Medicines scheme, the Department of Health has launched a bank of nearly 75,000 medical research volunteers to make it much easier for researchers to recruit people with specific conditions and a family history of conditions into research and trials.
The BioResource project, funded by the National Institute for Health Research (NIHR) and led from Addenbrooke’s Hospital, part of Cambridge University Hospital’, will focus on heart disease, dementia, infections and rare diseases and was developed as part of the UK’s commitment to life sciences.
Harpal Kumar, chief executive of Cancer Research UK, believes the new scheme could mean “the difference between life and death” as time is of the essence when it comes to cancer.
“Therefore this scheme, which has at its heart the potential to bring promising new medicines to patients faster, is to be warmly welcomed. The scheme should also make it more attractive for life sciences companies to conduct their development activities in the UK, which will bring a multitude of benefits to the population.”
Claire Halpin, of Empower: Access to Medicine, also welcomed the announcement and said: “There is no question that Les Halpin, my husband and founder of the Empower: Access to Medicine campaign, would have recognised this significant step forward on the road to ensuring those patients in desperate need have the opportunity to access treatments they could not in the past.
The pioneering Early Access to Medicines scheme will be funded by pharmaceutical companies which develop innovative treatments, meaning patients will benefit from world-class breakthroughs at no cost to the NHS. In return, the companies will be able to gain experience of their medicines being used in the NHS and work closely with regulators to look at the value of the drugs, gaining guidance and advice much earlier in the regulatory process.
As a result, the process of patient access will be speeded up and new drugs could be made available to patients months or sometimes years before the treatment is licensed.
Mr Hunt added: “Companies are expected to invest more in the UK as a result because of their increased confidence that they can work with doctors and patients to get experience of the new medicines and bring drugs to patients quicker. We expect charities and small businesses to also work together to develop new approaches to treating rare and life threatening diseases, which could include cancer, muscular dystrophy and dementia.”
Latest News Analysis
 04-Sep-19
Extra £1.5 billion announced for social care in Chancellor's Spending Review
04-Sep-19
Extra £1.5 billion announced for social care in Chancellor's Spending Review
 02-Jul-19
Department of Health forced to rethink care homes' nursing rates after legal challenge
02-Jul-19
Department of Health forced to rethink care homes' nursing rates after legal challenge
 18-Jun-19
Overnight care workers forced to sleep in offices and told 'bring your own bedding'
18-Jun-19
Overnight care workers forced to sleep in offices and told 'bring your own bedding'
 14-Jun-19
Back in the closet: Third of care home staff have had no LGBT+ awareness training
14-Jun-19
Back in the closet: Third of care home staff have had no LGBT+ awareness training
 11-Jun-19
PM candidates on social care: Rory Stewart calls fixing care an 'unfinished revolution'
11-Jun-19
PM candidates on social care: Rory Stewart calls fixing care an 'unfinished revolution'