Leading doctors claim NHS is 'over medicating' elderly patients with 'more medicine is better' culture
A group of high-profile doctors have called for an independent inquiry into the safety of medicines, after claiming NHS patients are being over medicated resulting in thousands of deaths every year.

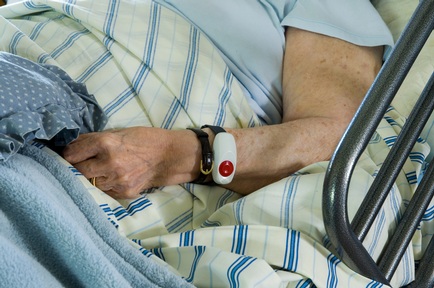
Older people are particularly vulnerable to polypharmacy – being prescribed four or more medications, with one in three hospital admissions in the over 75s a result of an adverse drug reaction.
The calls for greater transparency in the prescription of medicines come from the immediate past president of the Royal College of Physicians, Sir Richard Thompson, chair of the BMA General Practitioners committee, Dr Chaand Nagpaul, the president of the Faculty of Public Health, Professor John Ashton, chairman of the British Association of Physicians of Indian origin, consultant psychiatrist, Dr JS Bamrah and the editor in chief of JAMA Internal Medicine and Professor of Cardiology, Rita Redberg.
They are joined by leading NHS cardiologist, Dr Aseem Malhotra, who claims public funding is often allocated to medical research because it is likely to be profitable, not because it will be beneficial for patients.
“Without full transparency and accountability no doctor can provide what we slogged through medical school and devote our heart and souls to: providing the best quality care for our patients.
'Too much medicine'
“For the sake of our future health and the sustainability of the NHS it’s time for real collective action against ‘too much medicine’ starting with the Public Accounts Committee launching a full independent inquiry into the efficacy and safety of medicines.
“The underlying scandal that may ensue is likely to dwarf that of Mid Staffs. Medical science has taken a turn towards darkness. Sunlight will be its only disinfectant, he said in the Mail Online.
He claims that Britain faces an “epidemic of misinformed doctors and misinformed patients” due to biased research funding, biased reporting in medical journals and commercial conflicts of interest.
Prescription drugs 'third most common cause of death'
Citing recent studies, Dr Malhotra says that prescription drugs are the third most common cause of death after heart disease and cancer, with side effects of antidepressants and dementia drugs responsible for more than half a million deaths per year in the United States and Europe.
In relation to cholesterol lowering statin drugs he calls for a full reassessment of all the statin studies.
“Physicians should be aware that present claims about the efficacy and safety of statins are not evidence based,” he says, calling for the Clinical Trials Service Unit at Oxford University to release the raw data for independent scrutiny.
Dr Malhotra highlighted a number of cases where the National Institute of Clinical Excellence (NICE) and the drug regulator (the MHRA) have failed to manage lack of transparency and conflicts of interest over the prescription of several drugs including Tamiflu, Statins and Stroke drug alteplase.
Needs to be 'closer scrutiny'
Sir Richard Thompson, immediate past president of the Royal College of Physicians, commented: “Dr Malhotra again draws the attention of doctors and the public to the too often weak and sometimes murky basis on which the efficacy and use of drugs, particularly in the elderly, are judged. There needs to be closer scrutiny of the evidence underpinning drugs, and devices, and then better promotion of the evidence, together with more education of the public, doctors and medical students in how to assess the value of prescribing drugs to different groups of patients.
“The time has come for a full and open public enquiry into the way evidence of the efficacy of drugs is obtained and revealed. There is real danger that some current drug treatments are much less effective than had previously been thought.”
Professor John Ashton, president of the Faculty of Public Health, added: “Public health relies on a comprehensive, accurate and cost effective evidence base to ensure we make decisions based on the best available research that improve and protect people’s health, as well as prioritise care in the best way for patients. A public enquiry could be a useful tool in ensuring that research is published in a transparent and independent way.”
'Alarming increase of prescriptions in modern world'
Dr J S Bamrah, chairman of the British Association of Physicians of Indian origin, revealed that there has been an “alarming increase in prescriptions in the modern world which cannot be simply explained in terms of increasing disease”.
“The context of this in regards to the dangers of over-prescribing cannot be overstated. As Dr Aseem Malhotra rightly points out, there are a number of areas where there are incentives and conflicts that doctors and researchers have either been complicit or complacent, or plainly they have failed to declare their conflicts of interess. In some cases this will have led others to believe in their authoritative assertions,” he said.
Dr Bamrah has found in his own field of psychiatry “there are been much abuse and overuse of a number of drugs, and this pattern is destined to repeat unless someone like Dr Malhotra stands up to the establishment. His expose deserves a high level independent enquiry by the Government as otherwise patients will continue to rely on medications they need not have been prescribed by trusted doctors”.

Association of the British Pharmaceutical Industry
A spokesman for the Association of the British Pharmaceutical Industry responded to the claims, saying: “The pharmaceutical industry already works closely with the regulators to ensure safety and efficacy of branded medicines. The suggestion that prescription drugs are the third leading cause of death after cancer and heart disease is misleading and this is not a statistic recognised by either the World Health Organisation or the Office for National Statistics in the UK.
“The assessment of a medicine – the benefits and risks it brings to patients as well as the value it provides to healthcare – is an ongoing process. Innovating companies discover and develop new uses for these medicines over the life of these products, and regulators and health technology assessors continue to update their assessments based on new information. None of these procedures are “weak” or “murky” but by and large published for public scrutiny.
“However, we recognise that the discussion on the evaluation of medicines is timely, and we were pleased to contribute together with many other stakeholders to the ‘Evaluating Evidence’ policy programme of the Academy of Medical Sciences. This dialogue is critical to achieve a shared constructive and progressive framework for the assessment of medicines.”
Latest News Analysis
 04-Sep-19
Extra £1.5 billion announced for social care in Chancellor's Spending Review
04-Sep-19
Extra £1.5 billion announced for social care in Chancellor's Spending Review
 02-Jul-19
Department of Health forced to rethink care homes' nursing rates after legal challenge
02-Jul-19
Department of Health forced to rethink care homes' nursing rates after legal challenge
 18-Jun-19
Overnight care workers forced to sleep in offices and told 'bring your own bedding'
18-Jun-19
Overnight care workers forced to sleep in offices and told 'bring your own bedding'
 14-Jun-19
Back in the closet: Third of care home staff have had no LGBT+ awareness training
14-Jun-19
Back in the closet: Third of care home staff have had no LGBT+ awareness training
 11-Jun-19
PM candidates on social care: Rory Stewart calls fixing care an 'unfinished revolution'
11-Jun-19
PM candidates on social care: Rory Stewart calls fixing care an 'unfinished revolution'