Scientists reveal how protein build-up leads to Alzheimer's disease
A team of Cambridge researchers have identified that it may be possible to control how proteins build-up in the brain of someone with Alzheimer’s disease.
Researchers from the Department of Chemist at the University of Cambridge studied how amyloid plaques form and published the study in the journal, Nature Physics.
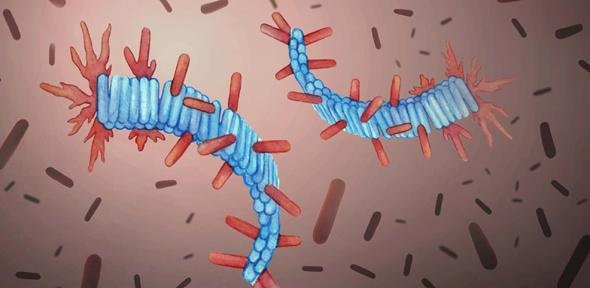
Scientists focused on amyloid plaque formation and discovered that after their long and slow build-up, the speed of their progression improves greatly, leading to the gradual decline witnessed in people living with Alzheimer’s disease.
First author of the study, Dr Andela Saric, explained: “One of the mysteries of amyloid plaque formation is how, after their long, slow formation, the speed of their progression becomes much faster. We’ve identified the factors that in fact cause the system to catalyse its own activity, becoming a runaway process. But this discovery suggests that if we’re able to control the build-up of healthy proteins on the fibrils, we might be able to limit the aggregation and spread of plaques.”
They found that similar to other biological molecules, such as DNA, the plaques had the ability to replicate themselves, however certain proteins were able to replicate without any additional assistance, such as the small, disease-causing protein fibres, fibrils, which are usually involved in neurodegenerative disorders including Alzheimer’s and Parkinson’s.
Dr Saric further argues that the findings could useful to those studying in the field of nanotechnology. She said: “One of the unfilled goals in nanotechnology is achieving efficient self-replication in manufacturing of nanomaterials. This is exactly what we’ve observed happening with these fibrils – if we’re able to learn the design rules from this process, we may be able to achieve this goal.”
During their study, researchers noticed that the fibrils, also known as amyloids, became intertwined with each other, causing the plaques that are found in the brains of people with Alzheimer’s. The formation of the first amyloid fibrils is very slow, and can take several decades, which could explain why Alzheimer’s often affects people in their old age. However, once the first fibrils are formed, they replicate and spread rapidly, making the disease unpredictable and difficult to manage.
Using a combination of computer simulations and laboratory experiments researchers were able to identify the necessary requirements for the self-replication of protein fibrils. They identified that the complicated process of fibril self-replication is governed by a physical mechanism – a build-up of healthy proteins on the surface of existing fibrils.
By using the molecule known as amyloid-beta, researchers were able to establish a relationship between the level of healthy proteins deposited on existing fibrils, and the rate of self-replication, highlighting the larger the build-up of proteins on fibrils, the faster they self-replicate.
They discovered that by changing how healthy proteins interact with the surface of fibrils, it is possible to control the fibril self-replication.
Head of research at Alzheimer's Society, Dr James Pickett, commented: “Reducing the build-up of amyloid plaques in the brain, the hallmark of Alzheimer's disease, is a key focus of several current clinical trials. However, there is an ongoing debate among scientists about which step in the process of amyloid plaque formation should be the target for drug treatments.
“Today's study brings more useful evidence to these discussions by using computer modelling to examine the interactions between amyloid proteins and predict what is happening in the brain of a person with Alzheimer’s when plaques are formed. The next step is for these predictions to be tested in living cells so that the information can usefully help in the design of more effective treatments to target amyloid.”
Latest News
 29-Jul-24
Dementia Bus gives carehome.co.uk staff insight into life with dementia
29-Jul-24
Dementia Bus gives carehome.co.uk staff insight into life with dementia
 01-Mar-24
Find out the top care homes in 2024
01-Mar-24
Find out the top care homes in 2024
 21-Mar-23
UK's top care homes in 2023 revealed
21-Mar-23
UK's top care homes in 2023 revealed
 03-Jan-23
carehome.co.uk launches free care helpline
03-Jan-23
carehome.co.uk launches free care helpline
 13-Dec-22
5 mins with Emily Whitehurst, chief operating officer for Constantia Healthcare
13-Dec-22
5 mins with Emily Whitehurst, chief operating officer for Constantia Healthcare